Vero Cell Vaccine Eu | Eu Starts Sinovac Jab Review Who Will Decide On Sinopharm This Week Cgtn
Recommendation for an Emergency Use Listing EUL of SINOVAC Covid-19 vaccine Vero cell Inactivated - CoronaVac TM Published. COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient.

Questions And Answers On Covid 19 Vaccination In The Eu European Commission
Vero 76 ATCC No.

Vero cell vaccine eu. COVID-19 vaccines under rolling review. Therefore providing them with the Janssen vaccine by Johnson Johnson would be the best choice. The vaccine is given by intramuscular injection into the deltoid muscle.
Sinovac COVID-19 vaccine Vero Cell Inactivated CoronaVac. Another labour destination in the region Oman is administering Pfizer-BioNTech and AstraZeneca vaccines. It is the also first vaccine that will carry a vaccine vial monitor a small sticker on the vaccine vials that change color as the vaccine is exposed to heat letting health workers know whether the vaccine can be safely used.
The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. It has not approved Vero Cell. One in the EU one produced by Serum Institute of India SII and the third by SKBio Republic of Korea on 15 February 2021 for emergency use as well as COVAX supply.
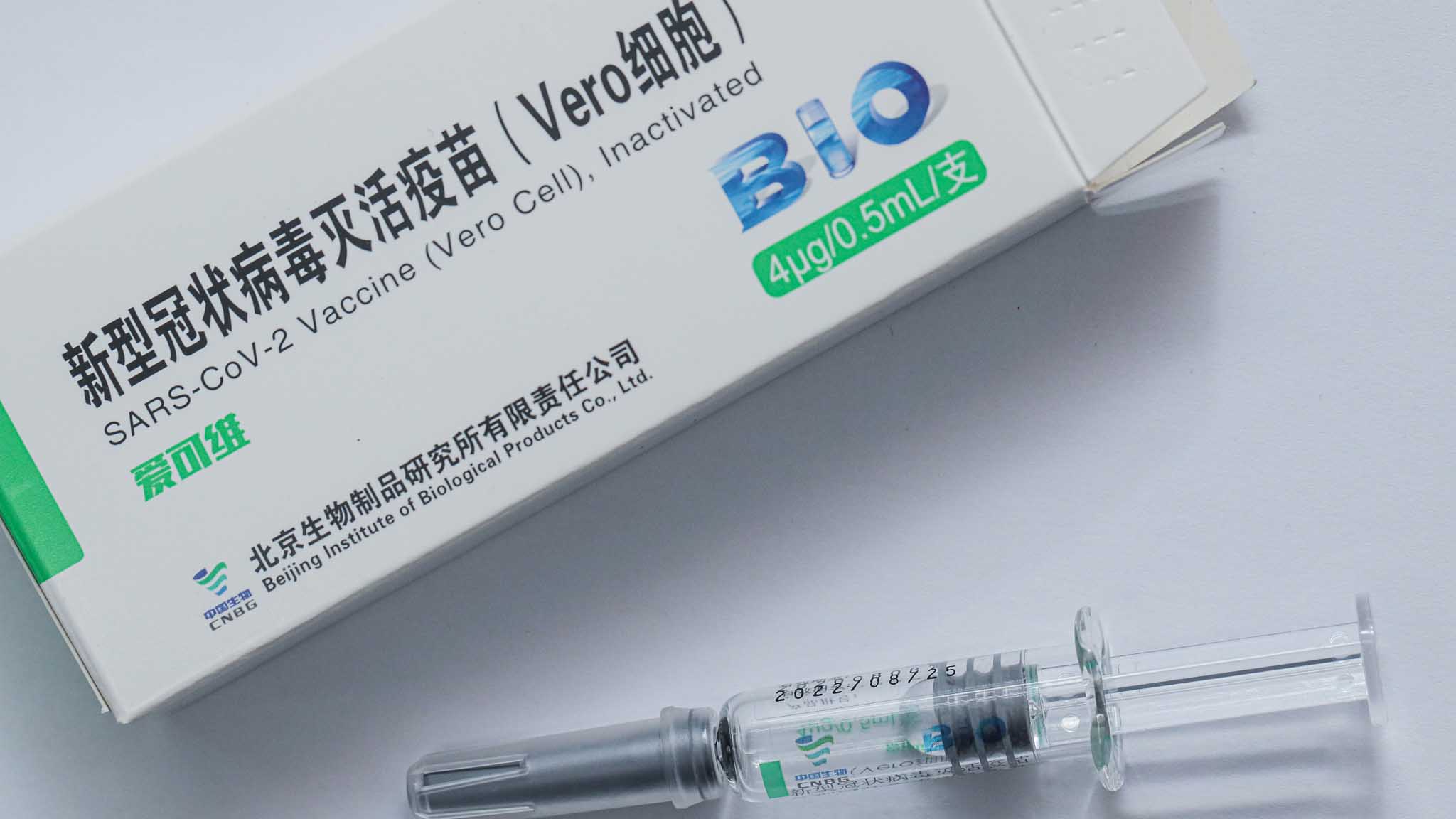
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. As a recent example CoronaVac COVID-19 vaccine developed by Sinovac Biotech uses vero cells in production and Vero term can be seen on the vaccine container. However on 4 May the European Union medicines regulator the European Medicines Agency EMA announced that it had started a rolling review of the Sinovac Vero Cell vaccine.
Any vaccine authorised for marketing by the European Union European Medicines Agency EMA and WHO will be accepted. EMA has initiated rolling reviews for the following COVID19 vaccines. The CHMP s decision to start the rolling review is based on preliminary results from laboratory studies non-clinical data and clinical.
The EU applicant for this medicine is LifeOn Srl. The vero lineage was isolated from kidney epithelial cells extracted. EMAs human medicines committee CHMP has started a rolling review of COVID-19 Vaccine Vero Cell Inactivated developed by Sinovac Life Sciences Co Ltd.
The company Sinopharm developed it in collaboration with the Wuhan Institute of Virology and the Institute of Biological Products. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension. Aethiops kidney on 27 Mar 1962.
As host cells for eukaryotic parasites specially of the trypanosomatids. Madagascars Minister of Health Professor Jean-Louis Rakotovao-Hanitrala said he was surprised and shocked to learn that the vaccines being distributed wouldnt be accepted in Europe. The World Health Organization WHO recommends an interval of 3 to 4 weeks between doses.
Its easy storage requirements make it highly suitable for low-resource settings. The vaccine is distributed by Novartis Vaccines and Diagnostics in Europe and the United States under the trade name IXIARO while in Australia the vaccine is licensed and distributed under the trade name JESPECT by CSL Biotherapies. The initial course consists of two doses and there is no evidence that a third booster dose dose is needed.
This vaccine was given to us by the WHO and welcomed by the European Union and all the UN agencies he was quoted as saying. Somarition August 12 2021. CCL-81 Isolated from C.
COVID-19 Vaccine Vero Cell Inactivated Sinovac - Advertisement - Yet according to the EU COVID-19 vaccine passport for travel regulation the Member States are obliged to issue and accept passports only to those who have been vaccinated with one of. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. Vero-Cell Inactivated SARS-CoV-2 Vero Cell Vaccine medicinal products not centrally authorized in the EU - 10 CoronaVac CoronaVa c Vaccine medicinal products not centrally authorized in the EU - 10 Covaxin Covaxin also known as BBV152 A B C Vaccine medicinal products not centrally authorized in the EU - 10 To be extended as vaccines are approved.
The complete vaccination schedule must be available for the vaccination certificate to be valid the official Travel Health Portal of Spain noted. Sinovac-CoronaVac Vero Cell Sinovac Life Sciences Co Ltd. CRL-1587 Isolated from Vero in 1968 it grows to a lower saturation density cells per unit area than the original Vero.
The European Medicines Agency EMA is evaluating potential COVID-19 vaccines to enable the distribution of promising vaccines in the European Union EU as soon as possible. WHO approved three versions of the AstraZeneca vaccine so far. Total doses administered in EUEEA countries 30 Reporting countries.
NVX-CoV2373 developed by Novavax rolling review started on 3 February 2021 CVnCoV by Curevac rolling review started on 12 February 2021 Sputnik V Gam-COVID-Vac by Gamaleya rolling review started on 4 March 2021 and COVID-19 Vaccine Vero Cell Inactivated by Sinovac Life Sciences Co Ltd rolling review. The chinese vaccine vero cell developed by sinopharm received emergency use approval from the world health organization on friday. The vaccine called Vero is based on an inactivated virus.
Inactivated Vero cell derived SA 14-14-2 vaccine is manufactured and licensed by Intercell AG Austria. Nepals major destination countries have mainly approved European and American vaccines said Shrestha. This is the first time that the who has approved vaccines produced outside europe and the united states.
Austria Belgium Bulgaria Croatia Cyprus Czechia Denmark Estonia Finland France Germany Greece Hungary Iceland Ireland Italy Latvia Lichtenstein Lithuania Luxembourg Malta Netherlands Norway Poland Portugal Romania Slovakia Slovenia Spain Sweden.
Paul Ehrlich Institut News Start Of The Rolling Review Procedure For Sinovac S Whole Virus Vaccine Called Covid 19 Vero Cell Inactivated At The European Medicines Agency

European Drugs Regulator Starts Rolling Review Of Sinovac Anti Coronavirus Jab

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

Ema Starts Rolling Review Of Covid 19 Vaccine Vero Cell Inactivated Eu Reporter
What Is Vero Cell Ema Launches Rolling Review Of Chinese Covid 19 Vaccine Euronews

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

Eu Starts Sinovac Jab Review Who Will Decide On Sinopharm This Week Cgtn
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian

Europe Starts Considering A Chinese Covid 19 Vaccine For Approval Fortune

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Hungary First In Eu To Innoculate With China S Sinopharm Vaccine Cgtn
Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
The Czech Republic Becomes The Second Eu Country To Ask For China S Sinopharm Vaccine

Travellers Vaccinated With Russian Chinese Indian Vaccines May Be Unable To Enter Majority Of Eu Countries Schengenvisainfo Com

Travellers Vaccinated With Russian Chinese Indian Vaccines May Be Unable To Enter Majority Of Eu Countries Schengenvisainfo Com
China S Sinopharm Vaccine Receives 1st Eu Gmp Certificate Cgtn

