Vero Cell Vaccine Efficacy / Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Real-world test-negative analysis in Bahrain based on 14 days post 2nd dose indicated a vaccine effectiveness of 90 95 CI 88 91 for adults aged 1859. Currently the IHME model uses the following inputs of vaccine efficacy separated by variant.

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
The efficacy of two doses of the vaccine.
Vero cell vaccine efficacy. What is the effectiveness of two doses of inactivated Vero cell-derived JE vaccine in preventing JE disease in individuals living in JE-endemic areas. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. As new data becomes available WHO.
Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies. The secondary objectives include evaluating the efficacy after at least one dose of the vaccine against RT-PCR confirmed symptomatic COVID-19 cases. The median duration of follow up available at the time of review was 112 days.
The primary objective is to evaluate the efficacy of an inactivated and aluminium hydroxide adsorbed SARS-CoV-2 vaccine Sinovac China in voluntary participants after 14 days of the second dose against RT-PCR confirmed symptomatic COVID-19 cases. Historically it is the first cell line that was approved by the WHO for the production of human vaccines. So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
Vaccine efficacy for symptomatic and hospitalized disease was estimated to be 79 all age groups combined. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases. Sinopharm vero cell vaccine efficacy.
Vaccine efficacy against hospitalization was 79. Assessments in settings where the P2 Variant of Concern was widely circulating also in Brazil - estimated vaccine effectiveness of 496 following at least one dose and demonstrated 507 two weeks after the second dose. Vero cell covid vaccine efficacy rate.
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace. On the basis of all available evidence WHO recommends the vaccine for adults 18 years and older in a two-dose schedule with a spacing of three to four weeks. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation.
Vero cell vaccine covid-19 efficacy rate. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning.
Sinopharm vero cell vaccine efficacy. Of studiesstarting rating 7 RCTs1 4 Factors decreasing confidence Limitation in. Rating Adjustment to rating t No.
Analysis was by intention to treat. KATHMANDU JUNE 25. Sinopharm vero cell vaccine efficacy.
Estimated Study Start Date. Interval of 21 days had the efficacy of 79 against symptomatic SARS-CoV-2 infection 14 days or more after the second dose. So far Vero is the only Chinese vaccine for which the manufacturer has published official data.
The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group. The study is registered with ClinicalTrialsgov number NCT00566345. Estimated Primary Completion Date.
The World Health Organization WHO has refuted the claims that a mix and match regimen of Vero Cell and AstraZeneca CoviShield vaccines. 1200SU vaccines for 28 days and then the positive seroconversion rate reached to over 941. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
The primary objective was the efficacy of the vaccine in preventing cell-culture-confirmed influenza infection with viruses that were antigenically matched to one of the vaccine strains. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. The above studies showed that the medium dose high dose and 014 and 028 procedures can produce a certain level of.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. A Multi-national Randomized Double-blind Placebo-controlled Phase III Clinical Study to Evaluate the Efficacy Safety and Immunogenicity of SARS-CoV-2 Vaccine Vero Cells Inactivated for the Prevention of COVID-19 in Healthy Adults Aged 18 Years and Older. The trial was not designed and powered to demonstrate efficacy against severe disease.

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
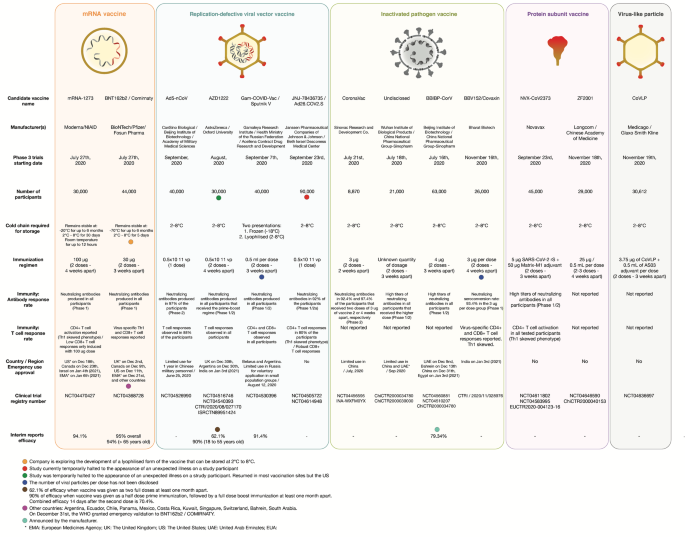
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
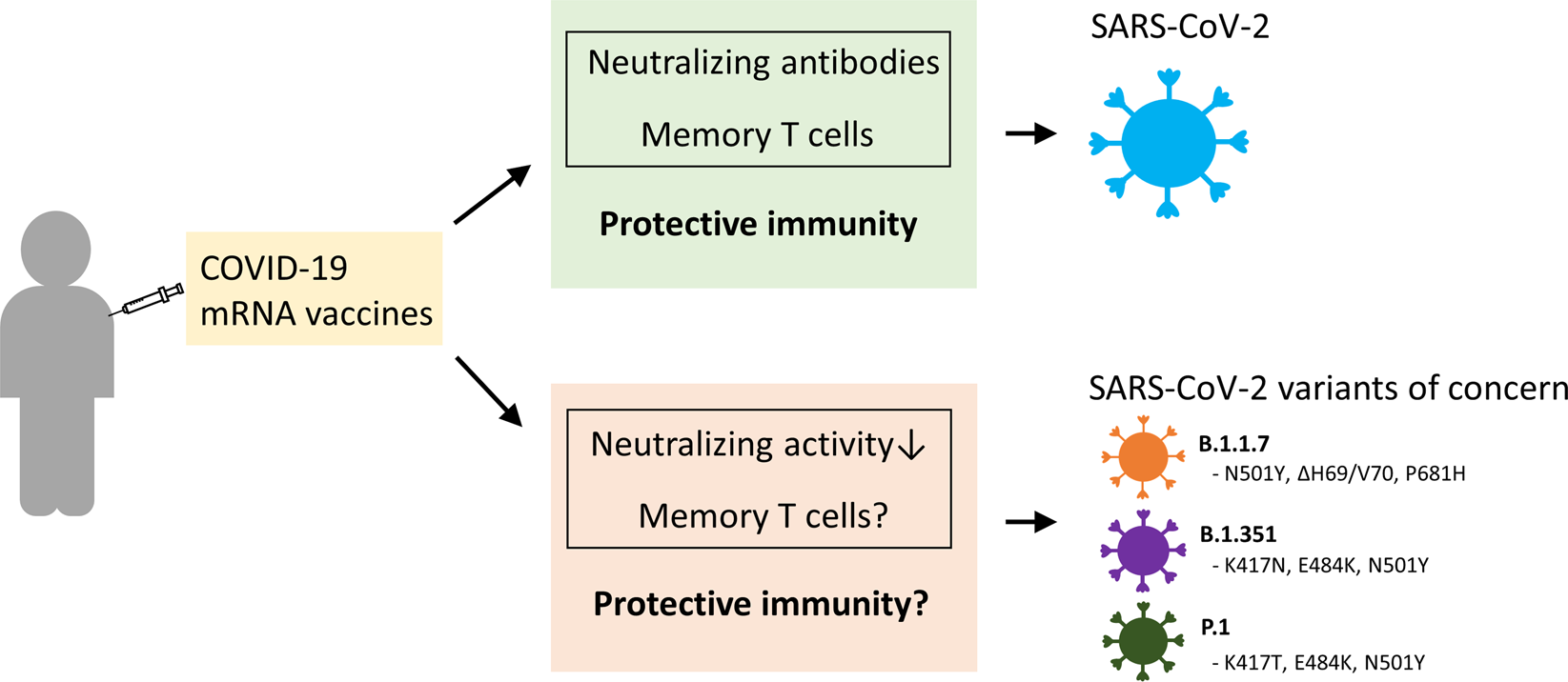
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
Https Www Who Int Immunization Policy Position Papers Je Grad Inactivated Effectiveness Pdf
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India


